Afluria
Afluria is a vaccine that is used to prevent the effects of influenza virus. Influenza virus develops every year and is getting stronger day by day. To cope with this dilemma, this vaccine has also been redeveloped every. It is improved by the health officer of that year.
Influenza is a very serious disease that is caused by a virus. It spreads very quickly. It effects on coming in contact a person suffering from it. More dangerous than that, it is widely spread by the saliva droplets that are expelled into the atmosphere whenever the affected person coughs or sneezes. Getting infected with influenza will be the last thing you want to happen to yourself. Influenza kills hundreds and hospitalizes thousands. It spreads quickly and causes deadly effects.
General Working
Afluria acts like a killed virus vaccine. It introduces your body with a specific virus that helps your body to build its immune system against the disease. It doesn’t actually treat any active infection that was previously developed in the body.
CPT CODE And Description
Q2035 – Influenza virus vaccine, split virus, when administered to individuals 3 years of age and older, for intramuscular use (afluria)
90656 – Influenza virus vaccine, trivalent (IIV3), split virus, preservative free, when administered to individuals 3 years and older, for intramuscular use
Vaccine Type CVX CPT
Effective January 1, 2017, redefined CPT codes 90656, 90657, 90658, 90685, 90686, 90687, and 90688 will reflect dosage amounts, in lieu of age indications.
1. Influenza IIV3 (inject) (multi-dose vials) 141 90658 (4 yrs & older)
2. Influenza IIV3 (P-Free, Inj) (prefilled syringe) 140 90656 (4 yrs & older)
3. Influenza IIV3 (Fluad, P-Free, Inj) (prefilled syringe) 168 90653 (65 yrs & older)
4. Influenza IIV3 High-Dose (prefilled syringe) 135 90662 (65 yrs & older)
5. Influenza LAIV4 (FluMist)* (prefilled nasal spray) 149 90672 (2-49yrs)
FREQUENCY OF ADMINISTRATION
Once per influenza season in the fall or winter Medicare may cover additional seasonal influenza virus vaccinations if medically necessary
Seasonal Influenza Virus Vaccine Administration Code: G0008 Diagnosis Code: V04.81
Effective for dates of service on or after October 1, 2010, HCPCS codes Q2035, Q2036, Q2037, Q2038 and Q2039 will replace the CPT code 90658 for Medicare payment purposes during the 2010 – 2011 influenza season, however, these HCPCS codes will not be recognized by the Medicare claims processing systems until January 1, 2011 when CPT code 90658 will no longer be recognized. Only the diagnoses codes and edits that are associated with CPT 90658 must also be applied to these new HCPCS codes.
Effective for dates of service on or after October 1, 2010, the Medicare Part B payment allowance in other situations for Q2036 is$8.784, for Q2037 is $13.253, and for Q2038 is $12.593. Since no national payment limits are available for Q2035 and Q2039, the payment limits will be determined by the local claims processing contractor.
General information
Afluria is available in different forms. The injectable form is actually killed virus vaccine as discussed above. Afluria is also available as nasal sprays which is the live virus vaccine. If you have any mild medical problem, then you can have Afluria vaccine, but if there is some serious infection or disease, then you must wait for it to come back to normal. You must also remember the problems and side effects that are caused by the Afluria vaccine because if you get to have Afluria vaccine, then you have to tell your doctor about the side effects.
Afluria vaccine cannot be considered as the universal relief. It cannot treat everyone. There are some restrictions and limitations of the vaccine. It doesn’t treat the illness caused by the bird flu. Afluria injects the same type of virus that causes the flu. But it doesn’t cause illness. However, one can feel flu in the season.
Receiving Afluria
Receiving Afluria treatment is a bit complicated procedure that one must practice extra precautions before receiving it. Here are some of the tips.
* First of all, you must have a complete record of the allergy or reaction caused by Afluria vaccination, if you were subjected to one before.
* Tell your doctor about these conditions for safely receiving Afluria vaccination
* Bone marrow transplant, Cancer treatment or any other medical treatment that can cause weakness in the immune system.
* Any neurological disorder in the history.
* If you are allergic to latex rubber
Afluria treatment
Afluria is given in two ways. The first is the injection into the muscle, and another is through nasal sprays. Injecting Afluria vaccine is the most common way of receiving Afluria. It consists of killed virus. You get an appointment with a doctor and get Afluria injected into your muscle. Afluria weakens your immune system so once you get the vaccination, you get addicted to it and must have it after every year. It is mostly carried out during November and October. Your doctor may advise you to get aspirin free medicine for pain and fever for 24 hours after the vaccination.
Missing a dose
The vaccination is carried out once in a year, so it is very easy to make an appointment and get your vaccination if you miss your dose. It is not too much of a worry, but you must get it as early as possible.
Side effects
Afluria doesn’t affect you with the flu virus that it injected into your body, but it can lead you to have the flu during the flu season. Keep the exact record of all the reactions caused by Afluria treatment. If you ever have to get Afluria treatment in future, you must have to tell them to your doctor. Never get a booster shot of Afluria if you had life-threatening side effects during the last time. Side effects demand an immediate reaction. Thus, you must call for a medical emergency if you feel any side effects like swelling of the face and lips, trouble breathing and hives after getting Afluria vaccination. Contact your doctor at the earliest hour if you experience any trouble after Afluria vaccination.
Following are some of the initial feelings you get if Afluria starts to affect you in an unwanted manner.
** Unusual bleeding
** Fever
** Convulsions
** Severe weakness or fatigue.
** Weakened arms and legs after two to four weeks of vaccination
** Here are some of the other side effects of Afluria
** Fussiness in mind
** Crying
** Redness and swelling on the part of the body where the vaccine was injected.
** A headache or weakness
** Muscle and joint pain
Dosage
The dosage of Afluria vaccination varies with age. Young people may get a stronger dose due to the strong immune system while the older people or infants are given a calculated amount of vaccination. Children having age below six months must not be subjected with the Afluria vaccination. Children of age more than the specified period must get a dose after four weeks. If a child gets a vaccination on day 1, then it must have the next shot after four weeks. Children of the age more than nine years must get 0.5 ml of Afluria vaccination. Adults must have a 0.5 ml of the vaccination in the deltoid muscle of the arm.
Specific conditions
Here are some specific medical conditions in which the vaccination must be carried out with ultimate care.
Pregnancy
It is still unknown that how Afluria affects the reproduction process. There are no studies that advocate the positive or negative role of Afluria on the pregnancy and reproduction capacity.
Nursing Mothers
Medicines get excreted in human milk. So extra precautions must be exercised while having an Afluria vaccination during nursing. There is no proper evidence whether it gets excreted or not.
Geriatric Application
Hem agglutination-inhibiting antibody reactions in the elderly people were significantly lower subsequent to the injection of Afluria as compared to young/adult subjects.
Furthermore, usage of Afluria vaccination for children under six months of age is not recommended. Children in this age group are most likely to get the side effects.
Presentation NDC Code CPT Code Q Code
Afluria Single-Use, Pre-filled Syringe 33332-016-01 90656 N/A
Afluria 5 mL Multi-Dose Vial 33332-116-10 90658 2035
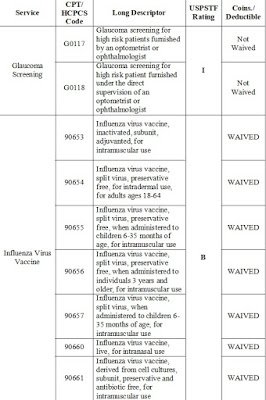
Medicare covered Influenza and Pneumococcal vaccines CPT Code Description
Medicare Covered for Influenza and Pneumococcal Vaccines listed below are eligible for Medicare Part B payment. These vaccines may be reimbursed regardless of the setting in which they are furnished. In addition, the administration fee for these vaccines is also eligible for payment.
90630 Influenza virus vaccine, quadrivalent (IIV4), split virus, preservative free, for intradermal use
90653 Influenza vaccine, inactivated (IIV), subunit, adjuvanted, for intramuscular use
90654 Influenza virus vaccine, trivalent (IIV3), split virus, preservative-free, for intradermal use
90655 Influenza virus vaccine, trivalent (IIV3), split virus, preservative free, 0.25 mL dosage, for intramuscular use
90656 Influenza virus vaccine, trivalent (IIV3), split virus, preservative free, 0.5 mL dosage, for intramuscular use
90657 Influenza virus vaccine, trivalent (IIV3), split virus, 0.25 mL dosage, for intramuscular use
90660 Influenza virus vaccine, trivalent, live (LAIV3), for intranasal use
90661 Influenza virus vaccine, trivalent (ccIIV3), derived from cell cultures, subunit, preservative and antibiotic free, 0.5 mL dosage, for intramuscular use
90662 Influenza virus vaccine (IIV), split virus, preservative free, enhanced immunogenicity via increased antigen content, for intramuscular use
90670 Pneumococcal conjugate vaccine, 13 valent (PCV13), for intramuscular use
90672 Influenza virus vaccine, quadrivalent, live (LAIV4), for intranasal use
90673 Influenza virus vaccine, trivalent (RIV3), derived from recombinant DNA,hemagglutinin (HA) protein only, preservative and antibiotic free, for intramuscular use
90674 Influenza virus vaccine, quadrivalent (ccIIV4), derived from cell cultures, subunit, preservative and antibiotic free, 0.5 mL dosage, for intramuscular use (Effective 07/01/2016)
90682 Influenza virus vaccine, quadrivalent (RIV4), derived from recombinant DNA, hemagglutinin (HA) protein only, preservative and antibiotic free, for intramuscular use
90685 Influenza virus vaccine, quadrivalent (IIV4), split virus, preservative free, 0.25 mL, for intramuscular use
90686 Influenza virus vaccine, quadrivalent (IIV4), split virus, preservative free, 0.5 mL dosage, for intramuscular use
90687 Influenza virus vaccine, quadrivalent (IIV4), split virus, 0.25 mL dosage, for intramuscular use
90688 Influenza virus vaccine, quadrivalent (IIV4), split virus, 0.5 mL dosage, for intramuscular use

